Which Filters To Use For Plga-pva Nanoparticles
Introduction
Constituting a astringent public health problem throughout the earth, the spectrum of leishmaniasis consists of neglected tropical diseases caused past parasite species of the genus Leishmania, 20 of which are capable of infecting humans (Masmoudi et al., 2013; Akhoundi et al., 2016; WHO, 2020a). Although endemic in 97 countries, the affliction is mainly full-bodied in Africa, Asia, and the Americas (WHO, 2020b). Information technology is currently estimated that around 12 meg people are infected worldwide, with an annual incidence of more than one million new cases per twelvemonth; one billion of the world's population lives in areas at chance of infection (Akhoundi et al., 2016; WHO, 2020a).
Leishmaniasis can be divided into ii primary clinical forms: cutaneous and visceral, with varying presentations depending on the species and virulence of the infecting parasite, likewise as the type of host immune response (Kaye and Scott, 2011; Akhoundi et al., 2016; Oryan and Akbari, 2016; Srivastava et al., 2016; Veras and De Menezes, 2016; WHO, 2020a). Amidst the cutaneous presentations, mucocutaneous leishmaniasis (MCL), caused mainly by Fifty. aethiopica in the Old World and L. braziliensis in the New Globe, is the most debilitating class, with subversive lesions occurring on the palate, lips and nasal septum (Akhoundi et al., 2016; Burza et al., 2018; WHO, 2020a). The most mutual form, localized cutaneous leishmaniasis (LCL), is caused past a diverseness of parasite species, including L. major, L. tropica, and 50. aethiopica in the Sometime Earth, in addition to Fifty. braziliensis, L. guyanensis, Fifty. amazonensis, and L. mexicana in the New World (Kaye and Scott, 2011; Masmoudi et al., 2013; Burza et al., 2018; Meira and Gedamu, 2019). Despite not being fatal, LCL tin can affect patients' quality of life according to the evolution and spread of skin lesions, social stigmatization, psychological effects, and absence (Carvalho et al., 1994; Scorza et al., 2017; Burza et al., 2018).
Currently, chemotherapy is the recommended treatment for patients diagnosed with leishmaniasis, mainly pentavalent antimonials and Amphotericin B in a complimentary or liposomal-encapsulated class (Croft et al., 2006; Frézard et al., 2009; Seifert, 2011; Brasil, 2015; De Menezes et al., 2015; Andrade-Neto et al., 2018). Alternatively, other drugs, such as pentamidine and paromomycin, tin too be practical in leishmaniasis handling (Santos et al., 2008; De Menezes et al., 2015). These therapies present several limitations, including high cost, invasive route of administration, prolonged cycle and systemic side effects, e.g., weakness, myalgia, rigors/chills, hemolysis and fever, as well every bit instability at high temperatures in some formulations (Sundar et al., 2004; Frézard et al., 2009; Chávez-Fumagalli et al., 2015; De Menezes et al., 2015). Additionally, drug aggregating in the organs can lead to pancreatitis, nephrotoxicity, hepatotoxicity, myocarditis, and cardiotoxicity (Rath et al., 2003; Frézard et al., 2009; Seifert, 2011; Masmoudi et al., 2013; Chávez-Fumagalli et al., 2015; De Menezes et al., 2015). The only currently bachelor not-invasive orally administered treatment for leishmaniasis, miltefosine, presents limitations including airsickness, diarrhea, kidney, and liver toxicity, every bit well as potential teratogenic furnishings and loftier cost in some regions (Rath et al., 2003; Seifert, 2011; Masmoudi et al., 2013; De Menezes et al., 2015; Andrade-Neto et al., 2018). This scenario highlights the need to discover new drugs that offer increased efficacy and less toxicity (De Menezes et al., 2015). So far, efforts to this end take focused on (i) increasing the condom and efficacy of treatments already in employ; (ii) combined drug therapy via novel therapeutic protocols; (iii) the search for new therapeutic targets in parasites or host cells; (4) repurposing drugs used to treat other diseases; (v) developing more effective commitment systems (Frézard et al., 2009; De Menezes et al., 2015; Andrade-Neto et al., 2018).
Heat Daze Protein 90 (Hsp90), a ubiquitous and highly conserved molecular chaperone, is responsible for performing the folding of other proteins, namely customer proteins, afterward preventing the mail-translational formation of oligomeric complexes with incorrect, inactive and not-functional structures (Zhao and Houry, 2005; Erlejman et al., 2014). This chaperone has been described every bit a potential therapeutic target in treating cancer and infectious diseases caused past different parasite species, including those of the Leishmania genus (Solit and Chiosis, 2008; Pallavi et al., 2010; Roy et al., 2012; Whitesell and Lin, 2012; Schopf et al., 2017; Guswanto et al., 2018). Notably, in leishmaniasis, Hsp90 has been shown to assist in re-establishing the functional stability of proteins in response to environmental force per unit area, such as differences in pH and temperature, during parasite differentiation processes (Zilberstein and Shapira, 1994; Graefe et al., 2002; Solit and Chiosis, 2008; Pallavi et al., 2010; Roy et al., 2012; Hombach et al., 2014; Schopf et al., 2017).
Structurally, Hsp90 is comprised of 3 main domains: the intermediate central proteolytic domain, involved in the interface between Hsp90 and its client proteins; the C-terminal domain, which facilitates homodimerization; the Northward-final domain, responsible for interaction with and the hydrolysis of ATP (Pratt and Toft, 2003; Zhao and Houry, 2005; Brown et al., 2007). The family unit of benzoquinone ansamycins constitutes a grade of Hsp90 inhibitors that compete with ATP for binding at the Hsp90 interaction site, thereby hindering chaperone activity (Zhao and Houry, 2005; Brown et al., 2007; Erlejman et al., 2014). Later, truncated or malformed proteins become degraded by the ubiquitin-proteasome arrangement (Chiosis et al., 2004; Xiao et al., 2006; Sidera and Patsavoudi, 2013). In parasites of the genus Leishmania, this inhibition leads to parasite death, evidencing the importance of Hsp90 in the maintenance of cellular homeostasis (Wiesgigl et al., 2001; Li et al., 2009; Roy et al., 2012; Hombach et al., 2013).
Enquiry by our group previously demonstrated that geldanamycin (GA), 17-AAG, and 17-DMAG, Hsp90 inhibitors of benzoquinone ansamycin family unit, were capable of eliminating promastigote forms of L. amazonensis at concentrations determined as non-toxic for human monocyte lineage cells (THP-i) (Palma et al., 2019). It was as well demonstrated that the treatment of Fifty. amazonensis-infected macrophages with 17-AAG reduced the per centum of infected macrophages and numbers of intracellular parasites in a time- and dose-dependent manner at concentrations deemed non-toxic to host cells (Petersen et al., 2012). Furthermore, 17-AAG was found to control, both in vitro and in vivo, 50. braziliensis infection in BALB/c mouse macrophages (Santos et al., 2014). This written report demonstrates that 17-AAG reduces the size of ear lesions and parasitic load at the lesion site, merely non in the draining lymph nodes of infected mice, resulting in infection relapse (Santos et al., 2014). To overcome this described limitation, it will exist tested a water-soluble analog of 17-AAG, 17-DMAG (Egorin et al., 2002; Sausville, 2004; Whitesell and Lin, 2012). Because it is a water-soluble molecule and has better pharmacokinetics than 17-AAG, 17-DMAG can achieve lymph nodes of treated animals, eliminating the parasites on this site. To optimize a formulation containing-Hsp90 inhibitor for leishmaniasis treatment, we propose the encapsulation of 17-DMAG in a nanoparticle, which can have controlled release of the drug, prolonging its action with fewer administrations. This commitment system can also help prevent toxicity occurrences in future tests for Fifty. braziliensis infection command.
Nanoparticle-based controlled release systems offer several advantages: improved safety, efficacy, target specificity (drug targeting), biocompatibility, bioavailability, biodegradability, and reduced toxicity in comparison to traditional drug delivery systems (Zhang et al., 2008; Formiga et al., 2009; Yildirimer et al., 2011; Lin, 2015; Utreja et al., 2020). By directing the active principle to specific tissues and releasing it gradually over time, the dose necessary to observe handling efficacy becomes reduced, thereby contributing to a reduction in side effects (Yildirimer et al., 2011; Wolfram et al., 2015). Synthetic polymers such every bit poly(lactic-co-glycolic acid) (PLGA), polylactic acid (PLA) and polycaprolactone (PCL) are commonly used in drug delivery systems because they exercise not offer a risk of inducing an unwanted allowed response (Formiga et al., 2009; Zhang and Zhang, 2017; Utreja et al., 2020). The main awarding of these systems consists of cases in which the gratuitous form of a drug presents limitations, such as shortened one-half-life, requiring the need for multiple applications, and inadequate target specificity, which tin lead to the occurrence of a range of side effects (Zhang et al., 2008; Formiga et al., 2009). Thus, the present work aimed to produce polymeric nanoparticles (NPs) containing the Hsp90 inhibitor, 17-DMAG, and perform morphological and physical-chemic characterization. The results obtained herein will enable, in the future, an evaluation of in vitro and in vivo optimized 17-DMAG-loaded NPs (NP2-17-DMAG) as antileishmanial treatment.
Materials and Methods
Reagents and Chemicals
The hydrochloride common salt of alvespimycin, 17-DMAG (Figure 1), was purchased from LC Laboratories (Massachusetts, USA). Resomer® RG 503 H, Poly (D, L-lactide-co-glycolide) (50:50) (PLGA), Poly (vinyl alcohol) (87–90% hydrolyzed average mol wt 30,000–70,000, PVA) and poly(ethylene glycol) (PEG, MW = eight,000) were purchased from Sigma-Aldrich (Darmstadt, Germany). Alamar blueish® was purchased from Invitrogen (Massachusetts, Usa). Microcon-thirty 0.5ML microtubes were purchased from Merk Millipore (Darmstadt, Germany). A C18 HPLC column and C18 Supelguard Guard Cartridge were purchased from Sigma-Aldrich (Darmstadt, Germany).
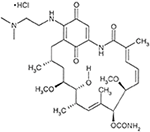
Figure 1. Chemical structure of 17-DMAG. Extracted from 17-DMAG datasheet (LC Laboratories).
Preparations of PLGA NPs
Two different double emulsion protocols were used to gear up PLGA NPs. In the first protocol (P1), PLGA NPs containing 17-DMAG (NP1-17-DMAG) were prepared using a modified double emulsion/solvent evaporation technique (Salvador et al., 2015). Briefly, l mg of PLGA was dissolved in five mL of acetone, and two mg of 17-DMAG was dissolved in five% PEG solution (250 mg in 5 mL of distilled h2o). The 17-DMAG solution was then added to the pre-cooled PLGA solution and emulsified using a Q700 sonicator (QSonica, Newtown, Connecticut, USA) at 6% aamplitude for ii min. Subsequently, ten mL of PVA one% w/v was added to the mixture. The recipient was and then covered with aluminum foil and and so homogenized at 10,000 rpm for 5 min (Ultra-turrax® T-25, IKA, Germany) to class a double emulsion. Next, x mL of 2% isopropyl booze was added. Finally, the suspension was magnetically stirred for 30 min with a subsequent solvent evaporation step using a rotary evaporator (IKA) for 1 h at 56°C under 250 mBar.
In the 2d double emulsion protocol (P2), PLGA NPs (NP2-17-DMAG) were also produced using another double emulsion/solvent evaporation technique (Mainardes et al., 2009). Initially, 100 mg of PLGA was dissolved in 4 mL of dichloromethane. Another solution containing ii mg of 17-DMAG dissolved in 1.five mL of PEG five% due west/v was then added to the pre-cooled PLGA solution to grade a single emulsion using a Q700 sonicator (QSonica, Newtown, Connecticut, USA) at 40% aamplitude for 1 min. To grade the double emulsion, the single emulsion was incorporated in 10 mL of PVA 1% w/v, then sonicated. Finally, 30 mL of PVA 1% w/v was added to the recipient, covered with aluminum foil and magnetically stirred for 30 min. The solvent extraction step was performed using a rotary evaporator (IKA) for one h at forty°C under 200 mBar.
Following each nanoparticle protocol training, suspensions were washed in distilled water three times at 39,800 × g for 15 min at four°C (T-865, Thermo Fisher Scientific, Massachusetts, Usa). Samples were frozen at −80°C, and so lyophilized for 24 h at −48°C nether 0.050 mBar (FreeZone 2.5 Liter Benchtop, Labconco, U.s.) and subsequently stored at 4°C. For blank nanoparticle training (NP1-Ø and NP2-Ø), these aforementioned protocols were followed in the absence of 17-DMAG.
To evaluate the influence of PLGA and PEG concentration on the encapsulation efficiency (%EE) of 17-DMAG and the size of the produced NPs, P2 was performed every bit described above using 100 or 200 mg of PLGA and 2.5% west/v or 5% w/v of PEG.
PLGA NPs Characterization
Dynamic Light Handful
DLS was used to mensurate the particle size, polydispersion index (PdI) and zeta potential (ZP) of the obtained NPs. After washing, NP-17-DMAG or NP-Ø produced by the P1 or P2 double-emulsion protocols were resuspended in 5 mL of distilled water and diluted one:125 in distilled water. DLS analysis was performed in triplicate using a ZetaSizer Nano ZS90 (Malvern Panalytical, UK) at 25°C.
Manual Electron Microscopy
For imaging and size confirmation, NPs were washed equally described in detail 2.ii and analyzed by TEM. Aliquots of NP-17-DMAG or NP-Ø produced by the P1 or P2 double-emulsion protocols were diluted 1:10 in distilled water, and so 10 μL of each sample was placed on a formvar film-coated filigree and stained with ii% uranyl acetate for 2 min. TEM analysis was performed using a JEM-1230 manual electron microscope (JEOL LTD, Nihon).
Scanning Electron Microscopy
SEM was used to examine the shape and surface morphology of the NP-17-DMAG or NP-Ø produced by the P1 or P2 double-emulsion protocols. For SEM analysis, lyophilized NPs (one mg) were placed on an adhesive stub and coated with gold-palladium under vacuum using an ion coater. All samples were analyzed and photographed at fifteen kV using a JSM-6390LV microscope (JEOL LTD, Japan).
HPLC for 17-DMAG Quantification
To quantify 17-DMAG, HPLC was performed using a C18 HPLC cavalcade and C18 Supelguard Guard Cartridge post-obit manufacturer protocols. Outset, the mobile phase was prepared using HPLC form acetonitrile (27%), HPLC course methanol (27%), ultrapure water (46%), and trifluoroacetic acid (0.05%). In parallel, 17-DMAG was diluted in distilled water at an initial concentration of 500 μg/mL, and so diluted from 50 to 1 μg/mL in the mobile phase to construct concentration curves in triplicate. Each sample was analyzed at a 2d wavelength of 254 nm for 8 min at 25°C. The mobile phase flow rate was 1 mL/min, with 10 μL of each sample injected. The observed retention time for 17-DMAG nether these weather condition was ~3.8 min. Nanoparticle concentrations of 17-DMAG were calculated using a free chemical compound bend (Empower software, version 3).
Encapsulation Efficiency (%EE) of 17-DMAG in NPs
Encapsulation efficiency determination was performed using two indirect methods: filter/column or supernatant. Afterward the solvent was evaporated using the filter/column method, 500 μL of nanoparticle break was centrifuged in a ane.5 mL microtube with a Microcon 30 filter under 14,000 × g for 1 h at iv°C. As the free drug fraction (F) in each sample flowed through the filter, NPs were retained. In parallel, accented ethanol was added to some other 500 μL aliquot of full nanoparticle intermission at a proportion of 1:1 to determine the total amount of 17-DMAG. After centrifugation at 6,200 × g for 15 min at 4°C, the supernatant was collected and the full quantity of 17-DMAG (T) was measured. %EE was evaluated by HPLC following the formula:
where T corresponds to the total mass of the drug in the sample, whether encapsulated or not, and F corresponds to the not-encapsulated fraction (free fraction).
For the supernatant method, the supernatants obtained from three washes of the produced NPs were nerveless and and then diluted at 1:5 in the mobile stage. HPLC so evaluated the %EE according to the formula:
where S corresponds to the total mass of 17-DMAG in the supernatants and T is the full mass of the drug added for encapsulation.
Release of 17-DMAG From NP2-17-DMAG in vitro
The release of 17-DMAG from NP2-17-DMAG was assessed in vitro using a modified method to determine the release kinetic profile (Quadros et al., 2020). Briefly, 2 mg of NP2-17-DMAG were placed into 1.5 mL polypropylene microcentrifuge tubes and resuspended in 1 mL of Dulbecco'southward modified Eagle'south medium (DMEM) (Gibco), supplemented with xx mM of HEPES (Sigma), 42.fourteen mM of sodium bicarbonate (Sigma), 10% of inactivated fetal bovine serum (Gibco), 2 mM of glutamine (Sigma), and 20 μg/mL of ciprofloxacin (Isofarma, Precabura, CE, BR) (complete DMEM medium). Next, the sealed tubes were placed in a rotating shaker and maintained at 37°C for 72 h. At each specific time signal (1, 3, vi, 12, 24, 48, and 72 h), the sample tubes were removed from the incubator and centrifuged at 21,000 × g for 15 min at 4°C. The supernatant was and so collected, frozen and immediately replaced with an equal volume of fresh release medium. To decide the 17-DMAG concentration, collected supernatants were diluted in the mobile phase and quantified using HPLC Shimadzu LC20-A (São Paulo, Brazil). All assays were performed in triplicate.
Animal Manipulation and Ethics Statement
BALB/c mice, male or female person, aged 6–12 weeks, were provided by the Gonçalo Moniz Found (IGM/FIOCRUZ) Beast Intendance Facility. The animals were maintained nether pathogen-gratuitous conditions, with food and water ad libitum. All procedures involving animals were conducted under the International Guiding Principles for Biomedical Research Involving Animals. The Institutional Review Lath approved this study's experimental design (CEUA protocol no. 007/2020) of the Gonçalo Moniz Institute, Bahia-Brazil (IGM–FIOCRUZ/BA).
Obtainment of Bone Marrow-Derived Macrophages From BALB/c Mice
BALB/c mice were euthanized using thiopental intraperitoneal injection (fifty mg/kg). Mouse femurs and tibias were aseptically removed and kept in cold 0.nine% NaCl solution containing 0.01 mg/mL of ciprofloxacin. In a sterile environment, bone extremities were removed and marrow cells were extracted past washing the bone cavity with Roswell Park Memorial Institute (RPMI) 1640 medium (GIBCO) supplemented with xx mM of HEPES (SIGMA), 23 mM of sodium bicarbonate (SIGMA), 10% of inactivated fetal bovine serum (Gibco), 2 mM of Glutamine (Sigma) and 20 μg/mL of ciprofloxacin (Isofarma, Precabura, CE, BR) (complete RPMI medium). Extracted marrow cells were centrifuged at 300 × g at 4°C for ten min, then resuspended and cultivated in Petri dishes (three plates per creature) containing 10 mL of complete RPMI medium with 30% supernatant from L929 cell civilization containing granulocyte macrophage colony-stimulating factor (GM-CSF). The dishes were cultivated at 37°C nether v% CO2 and 95% humidity for 24 h, after which the supernatant was transferred to new plates. After 72 h, an boosted 5 mL of consummate RPMI medium containing thirty% L929 supernatant was added to each civilization to re-stimulate cells for differentiation.
On the seventh day, BMDM were recovered from bacterial Petri dishes using 5 mL of 1 mM EDTA solution (pH 8.0) for v min at 37°C. Cells were centrifuged at 300 × thou for 10 min at 4°C, then resuspended in one mL of consummate DMEM medium and counted in a Neubauer chamber.
Uptake of Fluorescent NPs by BMDM in vitro
NPs containing rhodamine B (Sigma) were produced using the P2 protocol (detail 2.ii). BMDM were obtained as described to a higher place and plated at 105 cells per well in i mL of complete DMEM medium on 24-well plates containing glass coverslips. For the in vitro uptake analysis, rhodamine B-containing NPs were lyophilized and then incubated with BMDM for thirty min, 1, ii, iv, 6, 24, 48, and 72 h. Wells were done at each fourth dimension bespeak, and cells were stock-still with 4% paraformaldehyde (PFA) for xv min at room temperature. Finally, coverslips were mounted on slides using ProLong Gold antifade with DAPI® (Invitrogen, Darmstadt, Frg). Images were obtained by confocal fluorescence microscopy using a Leica SP8 device (Leica Microsystems, Mannheim, Frg).
Statistical Analysis
Graphs were constructed and statistical analyses were performed using GraphPad Prism version 5.01 (GraphPad Software Inc). The Kolmogorov-Smirnov examination was employed to verify normality. For data with Gaussian distribution, Student's t-test or one-mode ANOVA were used to compare between ii groups or among three or more groups, respectively, followed by Tukey'due south post-exam. For non-gaussian distributions, the Mann-Whitney U test was applied for comparisons between ii groups, while Kruskal-Wallis was used to compare iii or more groups. Differences were considered statistically pregnant when p < 0.05.
Results
DLS Label and %EE of NPs Produced by P1 or P2 Double Emulsion Protocols
The NP1-17-DMAG had a larger boilerplate size, size variation (PdI), and ZP than the NP2-17-DMAG (Table 1). No differences were detected in %EE values regardless of the quantitation method (supernatant or filter/column) used to determine the corporeality of 17-DMAG encapsulated in NP1-17-DMAG or NP2-17-DMAG (Table one).

Table 1. Particle size (Size), polydispersion index (PdI), zeta potential (ZP) and encapsulation efficiency (%EE) by supernatant of filter/column methods of NPs produced past P1 or P2 double emulsion protocols.
Morphological Label of NP1 and NP2
Consistent with the obtained PdI values, electron microscopy analysis revealed a more significant size variation in NP1 (Figures 2A,B) compared to NP2 (Figures 2C,D). Both protocols produced spherical, regular-shaped NPs (Figure 2). No morphological differences were observed betwixt NP-Ø and NP-17-DMAG.
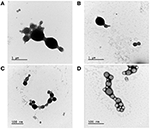
Figure two. Morphological characterization of NP1 and NP2 by TEM. Manual electron microscopy images of NP-Ø (A,C) and NP-17-DMAG (B,D) produced by P1 (A,B) or P2 (C,D) double emulsion protocols. 10 μL of each sample was contrasted with two% uranyl acetate for 2 min in a formvar grid. Bars stand for size references in μm or nm.
SEM morphological assay confirmed the spherical shape and shine surface of the NPs produced by both double emulsion protocols (Figure iii). Consistent with DLS and TEM results, SEM analysis also revealed that NP1-Ø and NP1-17-DMAG exhibited more considerable size variation (Figures 3A,B) compared to NP2-Ø and NP2-17-DMAG (Figures 3C,D). Again, no morphological differences were observed betwixt NP-Ø (Figures 3A,C) and NP-17-DMAG (Figures 3B,D) regardless of the protocol used.

Figure 3. SEM evaluations of NP1 and NP2. NP-Ø (A,C) or NP-17-DMAG (B,D) were produced by P1 (A,B) or P2 (C,D) double emulsion protocols. The produced NPs were frozen at −80°C and lyophilized for 24 h at −48°C under 0.050 mbar. Approximately i mg of each sample was vacuum-coated with gold-palladium using an ion coater and analyzed by SEM. All samples were analyzed and photographed at xv kV. Bars stand for 1 μm.
P2 Optimization Protocol
Every bit NP2-17-DMAG exhibited superior physical-chemical and morphological characteristics compared to NP1-17-DMAG, we employed P2 to optimize the 17-DMAG encapsulation process with some variations. NPs produced using 5% PEG presented a smaller median size of 297.2 nm (Q1: 288.6; Q2:304.1) compared to those made using 2.5% PEG (median size: 336.v nm; Q1: 318.4; Q2: 349.six) (Effigy 4A). Similarly, NPs produced containing 100 mg of PLGA presented a smaller median size of 336.five nm (Q1: 318.4; Q2: 349.6) in comparison to those containing 200 mg of PLGA (median size: 387.8 nm; Q1: 382.eight; Q2: 396.7) (Figure 4B). No differences were detected apropos PdI and ZP values amidst NPs prepared using different PEG concentrations (Figures 4C,E), nor different PLGA masses (Figures 4D,F).
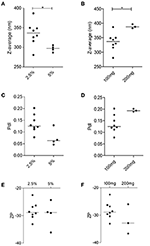
Figure 4. Evaluation of the influence of amount of PLGA and PEG concentration on nanoparticle physiochemical features in NP2-17-DMAG. DLS evaluated the effect of varying amounts of PEG (A,C,Due east) and PLGA (B,D,F) on the size (A,B), PdI (C,D), and ZP (E,F) of NP2-17-DMAG. Each signal represents an private experiment. Mann-Whitney test, *p < 0.05.
Similar %EE values were found for NP2-17-DMAG regardless of the amount of PEG used (ii.5 or 5%) (Figure 5A). Median %EE values for 2.5 and 5% of PEG were 23.51% (Q1: 19.66; Q2: 27.38) and 19.35% (Q1: 15.42; Q2: 42.eighteen), respectively, using the supernatant analysis method, vs. 34.12% (Q1: 29.31; Q2: 36.73) and 31.60% (Q1: 19.ninety; Q2: 48.79) using the filter/column method (Effigy 5A). Furthermore, similar %EE results were seen in NPs produced with different amounts of PLGA (Effigy 5B) using the supernatant quantitative assay method, with corresponding median values of 23.51% (Q1: 19.66; Q2: 27.38) and 26.48% (Q1: 18.78; Q2: 39.39) for 100 and 200 mg of PLGA, respectively. The filter/column method yielded median %EE values of 34.12% (Q1: 29.31; Q2: 36.73) for 100 mg and 32.12% (Q1: 24.66; Q2: 44.63) for 200 mg of this polymer. It is worth noting that, in comparison to the supernatant analysis method, higher %EE results were obtained using the quantitative filter/column method regardless of NP2 protocol modifications (Figure 5).
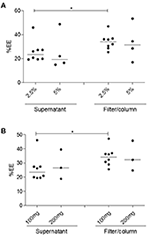
Effigy 5. Evaluation of the influence of PLGA and PEG concentration on the %EE of 17-DMAG in NP2-17-DMAG. The effect of varying amounts of PEG (A) and PLGA (B) on the %EE of 17-DMAG in NP2-17-DMAG was adamant by filter/column or supernatant analysis methods as described in section Material and Methods. Measurements were performed using HPLC. Each indicate represents an individual experiment. Isle of mann-Whitney test, *p < 0.05.
In vitro Release of 17-DMAG From NP2-17-DMAG
At one, three, 6, 12, 24, 48, and 72 h of incubation, five.36, 7.86, 9.85, xi.64, 13.41, 14.36, and 16% of 17-DMAG were cumulatively released from NP2-17-DMAG (Figure 6) in vitro, i.e., the amount of 17-DMAG was observed to continuously increase in consummate DMEM medium for 72 h (Effigy 6).
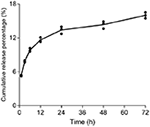
Effigy vi. Kinetics of 17-DMAG release from NP2-17-DMAG. NP2-17-DMAG were lyophilized and incubated in complete DMEM medium nether rotary agitation at 37°C. After 1, three, vi, 12, 24, 48 or 72 h, samples were centrifuged, the supernatant was collected, and the medium was replaced. The release of 17-DMAG was quantified in supernatants using HPLC. Mean, n = 3.
Uptake of Fluorescent NPs by BMDM in vitro
The rhodamine-encapsulated NPs produced by P2 (NP2-rhodamine) presented similar size, shape, and appearance (shine surface) equally the morphological characteristics of NPs produced with or without 17-DMAG (data not shown). The uptake of NP2-rhodamine past BMDM was observed at an early incubation time of thirty min, one, 2, 4, and 6 h (Figures 7A–E, respectively). Subsequently 24, 48 or 72 h (Figures 7F–H, respectively), greater numbers of NPs were observed in the cytoplasm of BMDM, indicating connected uptake by these cells.
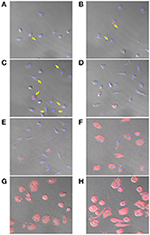
Figure 7. Uptake of NP2-rhodamine past BMDM. BMDM were plated at ten5 cells per well and incubated with NP2-rhodamine for 30 min (A), ane h (B), 2 h (C), 4 h (D), 6 h (E), 24 h (F), 48 h (G) or 72 h (H). Following incubation, cells were stock-still with 4% PFA and slides were mounted with DAPI. Cells were analyzed by confocal fluorescence microscopy (Leica SP8). Blue = DAPI; Cherry = rhodamine. Arrows bespeak internalization of NP2-rhodamine by macrophages at early on timepoints.
Give-and-take
The present piece of work encapsulated 17-DMAG in PLGA NPs and investigated the resulting physical-chemic, morphological, and biological parameters. To standardize NP production, two double emulsion-solvent evaporation protocols (P1 and P2) were used (Astete and Sabliov, 2006; Mainardes et al., 2009). Analyses past DLS, TEM and SEM showed that NP1 presented larger sizes and higher PdI than NP2, which agrees with a previous report (Astete and Sabliov, 2006). We found that modifications in the PLGA-NP production protocol, such as the nature of the organic solvent used or the emulsion method, altered the characteristics of the produced NP. The larger sizes presented past NP1 may exist due to laminar menses under stirring, such every bit that produced past the Ultra-Turrax® dispersing device. Appropriately, monodispersed drops may form in the emulsion, increasing the size of the produced NP, which is not observed under sonication (Astete and Sabliov, 2006). Concerning ZP, a measurement of the electric behavior of NPs, both protocols produced NPs with values around −30 mV, indicating stability with minimal aggregate germination (Formiga et al., 2009). Furthermore, the observed similarity between %EE values in each protocol can exist justified by using the same polymer and surfactant (PEG), as these parameters significantly influence the entrapment of hydrophilic drugs (Astete and Sabliov, 2006).
The morphological characterization of NP1 and NP2 past TEM and SEM confirmed differences in size and PdI between the two protocols, revealing a similarly regular, spherical shape and smooth surface advent. These aspects are essential every bit the morphological characteristics of NPs can predict possible interactions with living cells and uptake, intracellular localization and toxicity (Shang et al., 2014). Our results agree with previous studies (Cohen-Sela et al., 2009; McCall and Sirianni, 2013; Garbuzenko et al., 2014), demonstrating that PLGA particles produced by double emulsion presented spherical and regular shapes with smoothen surfaces, regardless of modifications performed in the protocol. Following DLS, TEM and SEM characterization, P2 was selected every bit the ameliorate protocol for NP-17-DMAG production.
We so evaluated the effects of modifications to P2: NP2-17-DMAG produced with 100 mg of PLGA presented smaller sizes than 200 mg. This effect stands in accordance with other authors who observed that increasing concentrations of PLGA generated larger particles (Astete and Sabliov, 2006; Rizkalla et al., 2006; Hernández-Giottonini et al., 2020). This has been observed to occur due to increased viscosity in the primary emulsion (westward/o), which results in a less-efficient particle size reduction during the double emulsification process (west/o/w) (Iqbal et al., 2015). Similarly, using five% PEG compared to 2.5% resulted in a smaller NP2-17-DMAG size. The decreased size was likewise observed at higher PEG concentrations in other studies (Zambaux et al., 1998; Rizkalla et al., 2006; Iqbal et al., 2015; Urbaniak and Musiał, 2019; Hernández-Giottonini et al., 2020). Higher surfactant concentrations reduce surface tension and promote particle division during the homogenization process, thus decreasing the size of the particles formed (Keum et al., 2011; Fonte et al., 2012). This phenomenon is maintained until a saturation point is reached (Keum et al., 2011; Fonte et al., 2012; Iqbal et al., 2015; Urbaniak and Musiał, 2019).
Encapsulation efficiency (%EE) was measured indirectly in this study. Two methods, filter/column or supernatant, were employed to separate loaded NPs and the non-encapsulated drug. We identified higher %EE values using filter/column than the supernatant, which may be attributable to superior gratis drug separation by this first method. This finding is consistent with studies on nanoparticle purification efficiency (Akbulut et al., 2012; Robertson et al., 2016), demonstrating that nanoparticle separation by supernatant depends on nanoparticle size and shape, the molecular weight of the polymer, dispersion medium density, speed, and centrifugation fourth dimension. The filter/column method has commonly been considered a more robust, more straightforward and efficient purification process (Robertson et al., 2016; Shah et al., 2020).
After characterizing NP2-17-DMAG, we evaluated the kinetics of drug release. We plant that NP2-17-DMAG exhibited two release phases: a preliminary rapid release of 17-DMAG lasting up to 24 h, followed by a wearisome and sustained release from 24 to 72 h. This data is similar to results obtained past Rietscher et al. (2016) and Rafiei and Azita (2017), who analyzed the release of different compounds in PLGA or PLGA-PEG NPs. PLGA NPs containing paromomycin also presented a similar release profile (Afzal et al., 2019), which was expected for polymeric NPs encapsulating hydrophilic molecules. At the initial timepoints evaluated, the drug is speedily released due to adsorbed molecules on the surface of NPs (Hirenkumar and Steven, 2012; Kapoor et al., 2015; Rietscher et al., 2016; Mir et al., 2017; Rafiei and Azita, 2017). In contrast, at subsequent time points, a sustained and slower release occurs due to gradual degradation of the polymeric matrix and slowly release of the drug independent at that place (Hirenkumar and Steven, 2012; Kapoor et al., 2015; Rietscher et al., 2016; Mir et al., 2017; Rafiei and Azita, 2017).
Nanoparticle uptake was evaluated through the incubation of BMDM with NP2-rhodamine. Fluorescence microscopy analysis revealed the internalization of NPs past macrophages beginning at early times of contact with NP2-rhodamine. At later timepoints, these particles continued to exist internalized and accumulation was observed in these cells' cytoplasm. These results are in agreement with other studies of professional phagocytes, including RAW 264 and J774 macrophage cell-lines and primary resident and inflammatory macrophages, which take been shown to internalize NPs at early timepoints, such as at 30 min of incubation (Cohen-Sela et al., 2009; Nicolete et al., 2011; Petersen et al., 2018; Couto et al., 2020; Van Hees et al., 2020).
The present report revealed that PLGA NPs containing 17-DMAG prepared using a double emulsion protocol presents concrete-chemical, morphological, and biological characteristics conducive to CL's treatment. Additional studies will exist carried out to investigate biological and immunological effects of NP2-17-DMAG in L. braziliensis infection control both in vitro and in vivo in a hereafter manuscript.
Information Availability Statement
The raw data supporting the conclusions of this article volition be made available by the authors, without undue reservation.
Ideals Statement
The beast study was reviewed and canonical past BALB/c mice, male or female, aged 6-12 weeks, were provided past the Gonçalo Moniz Institute (IGM/FIOCRUZ) Animal Intendance Facility. The animals were maintained under pathogen-free conditions, with food and water advertizing libitum. All procedures involving animals were conducted under the International Guiding Principles for Biomedical Research Involving Animals. The Institutional Review Board approved this report's experimental design (CEUA protocol no. 007/2020) of the Gonçalo Moniz Plant, Fiocruz, Salvador, Brazil.
Author Contributions
KC, BP, VP, FF, HR, and PV conceptualized and designed the experiments and analyzed and validated the data analysis. KC, BP, VP, MA, AP, DD, and MC performed the experiments. KC, VP, MA, AP, HQ, and PV wrote the paper. KC, BP, HR, FF, and PV reviewed and edited this paper. All authors contributed to the commodity and canonical the submitted version.
Funding
This work was supported by grants from the Bahia State Research Support Foundation (PV-Universal 422867/2016-0 and SUS0019/2014), FIOCRUZ (INOVA-46700287000), and Gonçalo Moniz Establish (PV-PROEP 400898/2013-6). PV holds a grant from CNPq (305235/2019-2). The fellowship received by KC was financed past Coordination for the Improvement of College Education Personnel-Brazil (CAPES)-Finance Code 001. This written report was partially supported past the Coordination for the Improvement of College Pedagogy Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES) - Finance Code 001. The funders had no role in study design, information collection or analysis, the decision to publish, or the manuscript'due south preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed equally a potential disharmonize of interest.
Acknowledgments
The authors would like to thank LAIPHE (FIOCRUZ–BA), LMN (Farmanguinhos–RJ), Electronic Microscopy Service (FIOCRUZ–BA), and Laboratory of Applied Chemistry Research (LIPAQ, SENAI CIMATEC–BA), for the back up in the experiment'due south performance. The authors besides would like to thank Carine South. A. Silva, Nathan A. A. A. Guiraud, and Gabriella B. Pita, for the realization of the initial experiments with polymeric nanoparticles, and Andris K. Walter, for English language language revision and manuscript copyediting help.
References
Afzal, I., Sarwar, H. S., Sohail, M. F., Varikuti, S., Jahan, Due south., Akhtar, S., et al. (2019). Mannosylated thiolated paromomycin-loaded PLGA nanoparticles for the oral therapy of visceral leishmaniasis. Nanomedicine fourteen, 387–406. doi: ten.2217/nnm-2018-0038
PubMed Abstract | CrossRef Full Text | Google Scholar
Akbulut, O., Mace, C. R., Martinez, R. V., Kumar, A. A., Nie, Z., Patton, M. R., et al. (2012). Separation of nanoparticles in aqueous multiphase systems through centrifugation. Nano Lett. 12, 4060–4064. doi: 10.1021/nl301452x
PubMed Abstruse | CrossRef Total Text | Google Scholar
Akhoundi, Chiliad., Kuhls, K., Cannet, A., Votýpka, J., Marty, P., Delaunay, P., et al. (2016). A historical overview of the nomenclature, evolution, and dispersion of leishmania parasites and sandflies. PLoS Negl. Trop. Dis. 10:e0004349. doi: 10.1371/journal.pntd.0004349
PubMed Abstract | CrossRef Full Text | Google Scholar
Andrade-Neto, V. V., Cunha-Inferior, E. F., Dos Santos Faioes, V., Martins, T. P., Silva, R. L., Leon, L. L., et al. (2018). Leishmaniasis treatment: update of possibilities for drug repurposing. Front. Biosci. Landmark 23, 967–996. doi: ten.2741/4629
PubMed Abstract | CrossRef Full Text | Google Scholar
Astete, C. East., and Sabliov, C. M. (2006). Synthesis and label of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 17, 247–289. doi: 10.1163/156856206775997322
CrossRef Total Text | Google Scholar
Brasil (2015). Transmission de recomendações para diagnóstico, tratamento e acompanhamento de pacientes com a coinfecção Leishmania-HIV, 1st Edn. Brasília: Ministério da Saúde.
Google Scholar
Brown, M. A., Zhu, 50., Schmidt, C., and Tucker, P. W. (2007). Hsp90-From signal transduction to cell transformation. Biochem. Biophys. Res. Commun. 363, 241–246. doi: 10.1016/j.bbrc.2007.08.054
PubMed Abstract | CrossRef Full Text | Google Scholar
Carvalho, Due east. M., Barral, A., Costa, J. M. 50., Bittencourt, A., and Marsden, P. (1994). Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop. 56, 315–325. doi: 10.1016/0001-706X(94)90103-1
PubMed Abstruse | CrossRef Full Text | Google Scholar
Chávez-Fumagalli, M. A., Ribeiro, T. G., Castilho, R. O., Fernandes, S. O. A., Cardoso, V. N., Coelho, C. S. P., et al. (2015). New delivery systems for amphotericin B applied to the improvement of leishmaniasis treatment. Rev. Soc. Bras. Med. Trop. 48, 235–242. doi: x.1590/0037-8682-0138-2015
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cohen-Sela, East., Chorny, M., Koroukhov, Due north., Danenberg, H. D., and Golomb, Chiliad. (2009). A new double emulsion solvent improvidence technique for encapsulating hydrophilic molecules in PLGA nanoparticles. J. Control. Release 133, ninety–95. doi: 10.1016/j.jconrel.2008.09.073
PubMed Abstract | CrossRef Total Text | Google Scholar
Couto, P. V., Magalhães, C. P., Ferrante, M., Rebouças, J., de, S., Nguewa, P., et al. (2020). Solid lipid nanoparticles as a novel conception approach for tanespimycin (17-AAG) against leishmania infections: Preparation, characterization and macrophage uptake. Acta Trop. 211:105595. doi: 10.1016/j.actatropica.2020.105595
PubMed Abstract | CrossRef Total Text | Google Scholar
Croft, Southward. Fifty., Seifert, K., and Yardley, 5. (2006). Current scenario of drug development for leishmaniasis. Indian J. Med. Res. 123, 399–410.
Google Scholar
De Menezes, J. P. B., Guedes, C. E. S., Petersen, A. L. O. A., Fraga, D. B. M., and Veras, P. Southward. T. (2015). Advances in development of new treatment for leishmaniasis. Biomed. Res. Int. 2015, 15–18. doi: x.1155/2015/815023
PubMed Abstract | CrossRef Full Text | Google Scholar
Egorin, M. J., Lagattuta, T. F., Hamburger, D. R., Covey, J. M., White, K. D., Musser, S. Thousand., et al. (2002). Pharmacokinetics, tissue distribution, and metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (NSC 707545) in CD 2f ane mice and fischer 344 rats. Cancer Chemother. Pharmacol. 49, 7–nineteen. doi: x.1007/s00280-001-0380-8
CrossRef Full Text | Google Scholar
Erlejman, A. Thousand., Lagadari, Thou., Toneatto, J., Piwien-Pilipuk, G., and Galigniana, M. D. (2014). Regulatory part of the 90-kDa-oestrus-stupor protein (Hsp90) and associated factors on gene expression. Biochim. Biophys. Acta Gene Regul. Mech. 1839, 71–87. doi: ten.1016/j.bbagrm.2013.12.006
PubMed Abstract | CrossRef Total Text | Google Scholar
Fonte, P., Soares, S., Costa, A., Andrade, J. C., Seabra, 5., Reis, Due south., et al. (2012). Effect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles afterward freeze-drying. Biomatter ii, 329–339. doi: x.4161/biom.23246
PubMed Abstruse | CrossRef Total Text | Google Scholar
Formiga, F. R., Ansorena, Eastward., De Mendonza, A. Eastward., Imbuluzqueta, E., González, D., and Prieto, Yard. J. B. (2009). Nanosistemas a base de poliésteres. Nanotecnología Farm. Existent. y posibilidades Farmacoter. Madrid: Existent Academia Nacional de Farmacia, 41–101.
Google Scholar
Garbuzenko, O. B., Winkler, J., Tomassone, K. S., and Minko, T. (2014). Biodegradable Janus nanoparticles for local pulmonary delivery of hydrophilic and hydrophobic molecules to the lungs. Langmuir 30, 12941–12949. doi: 10.1021/la502144z
PubMed Abstruse | CrossRef Total Text | Google Scholar
Graefe, S. E. B., Wiesgigl, Chiliad., Gaworski, I., Macdonald, A., and Clos, J. (2002). Inhibition of HSP90 in Trypanosoma cruzi induces a stress response but no stage differentiation. Eukaryot. Jail cell 1, 936–943. doi: 10.1128/EC.1.half-dozen.936-943.2002
PubMed Abstract | CrossRef Full Text | Google Scholar
Guswanto, A., Nugraha, A. B., Tuvshintulga, B., Tayebwa, D. Southward., Rizk, M. A., Batiha, G. E. South., et al. (2018). 17-DMAG inhibits the multiplication of several Babesia species and Theileria equi on in vitro cultures, and Babesia microti in mice. Int. J. Parasitol. Drugs Drug Resist. viii, 104–111. doi: 10.1016/j.ijpddr.2018.02.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Hernández-Giottonini, Grand. Y., Rodríguez-Córdova, R. J., Gutiérrez-Valenzuela, C. A., Peñuñuri-Miranda, O., Zavala-Rivera, P., Guerrero-Germán, P., et al. (2020). PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: effects of formulation parameters. RSC Adv. 10, 4218–4231. doi: 10.1039/C9RA10857B
CrossRef Full Text | Google Scholar
Hirenkumar, M., and Steven, South. (2012). Poly Lactic-co-Glycolic Acrid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 3, 1377–1397. doi: 10.3390/polym3031377
PubMed Abstract | CrossRef Full Text | Google Scholar
Hombach, A., Ommen, G., Chrobak, M., and Clos, J. (2013). The Hsp90-Sti1 interaction is disquisitional for Leishmania donovani proliferation in both life cycle stages. Cell. Microbiol. 15, 585–600. doi: 10.1111/cmi.12057
PubMed Abstruse | CrossRef Total Text | Google Scholar
Hombach, A., Ommen, G., MacDonald, A., and Clos, J. (2014). A small heat stupor protein is essential for thermotolerance and intracellular survival of Leishmania donovani. J. Cell Sci. 127, 4762–4773. doi: 10.1242/jcs.157297
PubMed Abstract | CrossRef Full Text | Google Scholar
Iqbal, 1000., Valour, J. P., Fessi, H., and Elaissari, A. (2015). Preparation of biodegradable PCL particles via double emulsion evaporation method using ultrasound technique. Colloid Polym. Sci. 293, 861–873. doi: ten.1007/s00396-014-3464-ix
CrossRef Total Text | Google Scholar
Kapoor, D. Due north., Bhatia, A., Kaur, R., Sharma, R., Kaur, One thousand., and Dhawan, South. (2015). PLGA: a unique polymer for drug commitment. Ther. Deliv. half dozen, 41–58. doi: 10.4155/tde.14.91
PubMed Abstruse | CrossRef Total Text | Google Scholar
Kaye, P., and Scott, P. (2011). Leishmaniasis: complication at the host-pathogen interface. Nat. Rev. Microbiol. 9, 604–615. doi: 10.1038/nrmicro2608
CrossRef Full Text | Google Scholar
Keum, C. G., Noh, Y. W., Baek, J. S., Lim, J. H., Hwang, C. J., Na, Y. 1000., et al. (2011). Practical preparation procedures for docetaxel-loaded nanoparticles using polylactic acid-co-glycolic acid. Int. J. Nanomedicine half dozen, 2225–2234. doi: 10.2147/ijn.s24547
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, Q., Zhou, Y., Yao, C., Ma, X., Wang, L., Xu, W., et al. (2009). Apoptosis caused past Hsp90 inhibitor geldanamycin in Leishmania donovani during promastigote-to-amastigote transformation stage. Parasitol. Res. 105, 1539–1548. doi: 10.1007/s00436-009-1582-y
PubMed Abstruse | CrossRef Full Text | Google Scholar
Mainardes, R. Thousand., Gremião, Chiliad. P. D., Brunetti, I. L., Fonseca, L. Chiliad., and Khalil, N. One thousand. (2009). Zidovudine-loaded PLA and PLA–PEG blend nanoparticles: influence of polymer blazon on phagocytic uptake by polymorphonuclear cells. Pharm. Nanotechnol. 98, 257–267. doi: x.1002/jps.21406
PubMed Abstruse | CrossRef Total Text | Google Scholar
Masmoudi, A., Hariz, West., Marrekchi, S., Amouri, M., and Turki, H. (2013). Quondam earth cutaneous leishmaniasis: diagnosis and handling. J. Dermatol. Case Rep. vii, 31–41. doi: 10.3315/jdcr.2013.1135
PubMed Abstruse | CrossRef Full Text | Google Scholar
Mir, M., Ahmed, N., and Rehman, A. (2017). Recent applications of PLGA based nanostructures in drug commitment. Colloids Surf. B Biointerfaces 159, 217–231. doi: 10.1016/j.colsurfb.2017.07.038
PubMed Abstract | CrossRef Total Text | Google Scholar
Nicolete, R., Santos, D. F. D., and Faccioli, Fifty. H. (2011). The uptake of PLGA micro or nanoparticles past macrophages provokes distinct in vitro inflammatory response. Int. Immunopharmacol. xi, 1557–1563. doi: 10.1016/j.intimp.2011.05.014
PubMed Abstruse | CrossRef Full Text | Google Scholar
Oryan, A., and Akbari, M. (2016). Worldwide hazard factors in leishmaniasis. Asian Pac. J. Trop. Med. 9, 925–932. doi: 10.1016/j.apjtm.2016.06.021
CrossRef Full Text | Google Scholar
Pallavi, R., Roy, N., Nageshan, R. K., Talukdar, P., Pavithra, Due south. R., Reddy, R., et al. (2010). Heat stupor poly peptide ninety equally a drug target against protozoan infections: biochemical characterization of HSP90 from plasmodium falciparum and Trypanosoma evansi and evaluation of its inhibitor as a candidate drug. J. Biol. Chem. 285, 37964–37975. doi: 10.1074/jbc.M110.155317
PubMed Abstract | CrossRef Full Text | Google Scholar
Palma, Fifty. C., Ferreira, L. F. G. R., Petersen, A. Fifty., de, O. A., Dias, B. R. S., Menezes, J. P. B., and de Moreira,, D. R.. (2019). A docking-based structural analysis of geldanamycin-derived inhibitor binding to human being or Leishmania Hsp90. Sci. Rep. 9:14756. doi: x.1038/s41598-019-51239-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Petersen, A. L., de, O. A., Campos, T. A., Santos Dantas, D. A., dos Rebouças, J., de, S., and da Silva, J. C. .(2018). Encapsulation of the HSP-90 chaperone inhibitor 17-AAG in stable liposome let increasing the therapeutic alphabetize every bit assessed, in vitro, on Leishmania (L) amazonensis amastigotes-hosted in mouse CBA macrophages. Front. Cell. Infect. Microbiol. viii, 1–xiv. doi: x.3389/fcimb.2018.00303
PubMed Abstract | CrossRef Full Text | Google Scholar
Petersen, A. L., de, O. A., Guedes, C. Eastward. Southward., Versoza, C. L., Lima, J. One thousand. B., de Freitas, 50. A. R., et al. (2012). 17-AAG Kills intracellular Leishmania amazonensis while reducing inflammatory responses in infected macrophages. PLoS I 7:e49496. doi: x.1371/journal.pone.0049496
PubMed Abstruse | CrossRef Total Text | Google Scholar
Pratt, Due west. B., and Toft, D. O. (2003). Regulation of signaling protein role and trafficking past the hsp90/hsp70-based chaperone mechanism. Exp. Biol. Med. 228, 111–133. doi: 10.1177/153537020322800201
PubMed Abstract | CrossRef Total Text | Google Scholar
Quadros, H. C., Santos, Fifty., de, K. F., Meira, C. S., Khouri, M. I., Mattei, B., et al. (2020). Development and in vitro characterization of polymeric nanoparticles containing recombinant adrenomedullin-2 intended for therapeutic angiogenesis. Int. J. Pharm. 576:118997. doi: 10.1016/j.ijpharm.2019.118997
PubMed Abstract | CrossRef Full Text | Google Scholar
Rafiei, P., and Azita, H. (2017). Docetaxel-loaded Plga and Plga-Peg nanoparticles for intravenous awarding: pharmacokinetics and biodistribution profile. Int. J. Nanomed. 12, 935–947. doi: ten.2147/IJN.S121881
PubMed Abstruse | CrossRef Full Text | Google Scholar
Rath, S., Augusto Trivelin, L., Imbrunito, T. R., Tomazela, D. M., De Jesús, M. Due north., Calvo Marzal, P., et al. (2003). Antimoniais empregados no tratamento da leishmaniose: Estado da arte. Quim. Nova 26, 550–555. doi: 10.1590/S0100-40422003000400018
CrossRef Full Text | Google Scholar
Rietscher, R., Czaplewska, J. A., Majdanski, T. C., Gottschaldt, Grand., Schubert, U. S., Schneider, Grand., et al. (2016). Impact of PEG and PEG-b-PAGE modified PLGA on nanoparticle formation, protein loading and release. Int. J. Pharm. 500, 187–195. doi: ten.1016/j.ijpharm.2016.01.021
PubMed Abstract | CrossRef Full Text | Google Scholar
Rizkalla, N., Range, C., Lacasse, F. 10., and Hildgen, P. (2006). Consequence of various formulation parameters on the backdrop of polymeric nanoparticles prepared by multiple emulsion method. J. Microencapsul. 23, 39–57. doi: ten.1080/02652040500286185
PubMed Abstract | CrossRef Full Text | Google Scholar
Robertson, J. D., Rizzello, L., Avila-Olias, G., Gaitzsch, J., Contini, C., Magoń, One thousand. South., et al. (2016). Purification of Nanoparticles by Size and Shape. Sci. Rep. vi, 1–9. doi: x.1038/srep27494
CrossRef Full Text | Google Scholar
Roy, N., Nageshan, R. K., Ranade, S., and Tatu, U. (2012). Heat shock protein 90 from neglected protozoan parasites. Biochim. Biophys. Acta Mol. Cell Res. 1823, 707–711. doi: 10.1016/j.bbamcr.2011.12.003
PubMed Abstract | CrossRef Full Text | Google Scholar
Salvador, A., Sandgren, K. J., Liang, F., Thompson, East. A., Koup, R. A., Pedraz, J. 50., et al. (2015). Design and evaluation of surface and adjuvant modified PLGA microspheres for uptake by dendritic cells to improve vaccine responses. Int. J. Pharm. 496, 371–381. doi: 10.1016/j.ijpharm.2015.10.037
PubMed Abstruse | CrossRef Full Text | Google Scholar
Santos, D. M., Petersen, A. Fifty. O. A., Celes, F. S., Borges, V. M., Veras, P. S. T., and de Oliveira, C. I. (2014). Chemotherapeutic potential of 17-AAG confronting cutaneous Leishmaniasis acquired by Leishmania (Viannia) braziliensis. PLoS Negl. Trop. Dis. 8:e3275. doi: 10.1371/journal.pntd.0003275
PubMed Abstract | CrossRef Full Text | Google Scholar
Santos, D. O., Coutinho, C. E. R., Madeira, M. F., Bottino, C. Chiliad., Vieira, R. T., Nascimento, S. B., et al. (2008). Leishmaniasis handling—a challenge that remains: a review. Parasitol. Res. 103, 1–10. doi: 10.1007/s00436-008-0943-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Schopf, F. H., Biebl, G. M., and Buchner, J. (2017). The HSP90 chaperone mechanism. Nat. Rev. Mol. Cell Biol. 18, 345–360. doi: 10.1038/nrm.2017.20
CrossRef Total Text | Google Scholar
Shah, Northward. K., Ivone, R., Shen, J., and Meenach, South. A. (2020). A comparison of centrifugation and tangential flow filtration for nanoparticle purification: a instance study on acetalated dextran nanoparticles. Particuology 50, 189–196. doi: 10.1016/j.partic.2019.06.004
CrossRef Full Text | Google Scholar
Sidera, K., and Patsavoudi, E. (2013). HSP90 inhibitors: current development and potential in cancer therapy. Recent Pat. Anticancer. Drug Discov. 9, 1–20. doi: 10.2174/15748928113089990031
PubMed Abstruse | CrossRef Full Text | Google Scholar
Solit, D. B., and Chiosis, G. (2008). Development and application of Hsp90 inhibitors. Drug Discov. Today 13, 38–43. doi: 10.1016/j.drudis.2007.10.007
CrossRef Full Text | Google Scholar
Srivastava, S., Shankar, P., Mishra, J., and Singh, Southward. (2016). Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasit. Vectors 9:277. doi: 10.1186/s13071-016-1553-y
PubMed Abstruse | CrossRef Full Text | Google Scholar
Sundar, South., Mehta, H., Suresh, A. V., Singh, Due south. P., Rai, Grand., and Murray, H. W. (2004). Amphotericin B handling for Indian visceral leishmaniasis: conventional versus lipid formulations. Clin. Infect. Dis. 38, 377–383. doi: x.1086/380971
PubMed Abstruse | CrossRef Full Text | Google Scholar
Urbaniak, T., and Musiał, W. (2019). Influence of solvent evaporation technique parameters on bore of submicron lamivudine-poly-ε-caprolactone cohabit particles. Nanomaterials nine:1240. doi: 10.3390/nano9091240
PubMed Abstract | CrossRef Full Text | Google Scholar
Utreja, P., Verma, S., Rahman, M., and Kumar, 50. (2020). Utilize of nanoparticles in medicine. Curr. Biochem. Eng. six, 7–24. doi: ten.2174/2212711906666190724145101
CrossRef Full Text | Google Scholar
Van Hees, S., Elbrink, Grand., De Schryver, K., Delputte, P. Fifty., and Kiekens, F. (2020). Improving cellular uptake and cytotoxicity of chitosan-coated poly(lactic- co -glycolic acid) nanoparticles in macrophages. Nanomedicine fifteen, 2671–2688. doi: x.2217/nnm-2020-0317
PubMed Abstract | CrossRef Full Text | Google Scholar
Veras, P. S. T., and De Menezes, J. P. B. (2016). Using proteomics to understand how Leishmania parasites survive inside the host and establish infection. Int. J. Mol. Sci. 17:1270. doi: 10.3390/ijms17081270
PubMed Abstract | CrossRef Full Text | Google Scholar
Whitesell, L., and Lin, North. U. (2012). HSP90 as a platform for the assembly of more effective cancer chemotherapy. Biochim. Biophys. Acta Mol. Cell Res. 1823, 756–766. doi: 10.1016/j.bbamcr.2011.12.006
PubMed Abstract | CrossRef Total Text | Google Scholar
WHO (2020b). Weekly Epidemiological Tape. Geneva: WHO, 265–280.
Google Scholar
Wiesgigl, Thousand., Clos, J., and Schekman, R. W. (2001). Heat shock protein 90 homeostasis controls stage differentiation in Leishmania donovani. Mol. Biol. Cell 12, 3307–3316. doi: 10.1091/mbc.12.11.3307
PubMed Abstract | CrossRef Full Text | Google Scholar
Wolfram, J., Zhu, M., Yang, Y., Shen, J., Gentile, Eastward., Paolino, D., et al. (2015). Condom of nanoparticles in medicine. Curr. Drug Targets 16, 1671–1681. doi: x.2174/1389450115666140804124808
CrossRef Total Text | Google Scholar
Yildirimer, L., Thanh, North. T. 1000., Loizidou, M., and Seifalian, A. Yard. (2011). Toxicological considerations of clinically applicable nanoparticles. Nano Today vi, 585–607. doi: 10.1016/j.nantod.2011.x.001
CrossRef Total Text | Google Scholar
Zambaux, M. F., Bonneaux, F., Gref, R., Maincent, P., Dellacherie, Due east., Alonso, M. J., et al. (1998). Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. J. Control. Release l, 31–forty. doi: x.1016/S0168-3659(97)00106-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, L., Gu, F. 10., Chan, J. M., Wang, A. Z., Langer, R. S., and Farokhzad, O. C. (2008). Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 83, 761–769. doi: 10.1038/sj.clpt.6100400
PubMed Abstruse | CrossRef Total Text | Google Scholar
Zhang, X., and Zhang, P. (2017). Polymersomes in nanomedicine–a review. Curr. Nanosci. 13, 124–129. doi: x.2174/1573413712666161018144519
CrossRef Full Text | Google Scholar
Zilberstein, D., and Shapira, M. (1994). The role of pH and temperature in the development of leishmania parasites. Annu. Rev. Microbiol. 48, 449–470. doi: 10.1146/annurev.mi.48.100194.002313
PubMed Abstract | CrossRef Full Text | Google Scholar
Which Filters To Use For Plga-pva Nanoparticles,
Source: https://www.frontiersin.org/articles/644827
Posted by: morrisonthaven.blogspot.com


0 Response to "Which Filters To Use For Plga-pva Nanoparticles"
Post a Comment